Clinical Case: Is it possible to do more with less? Cracking the chameleon effect
Using 3M™ Filtek™ Easy Match Universal Restorative.


You may use this form to provide the information and photos needed for your clinical case. Depending on your case, you may not need all the photo areas.
We only accept Clinical Cases from Health Care Professionals (HCP) in EMEA (Europe, Middle East, Africa). The content must be in English.
Your professional information provided below, will be used to respond to your request and as further described in our Internet Privacy Policy.
Refer to Instructions for Use (IFU) for complete product information. Results may vary.
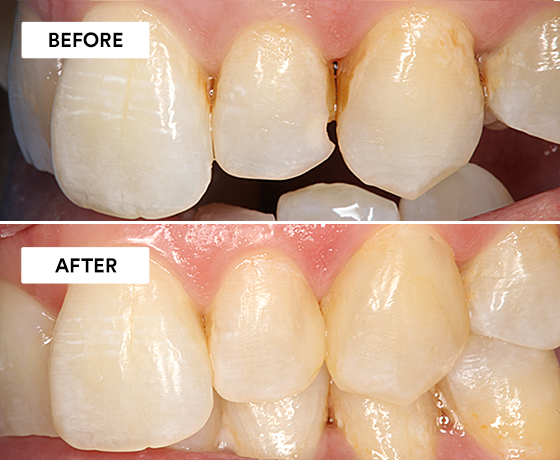
Using 3M™ Filtek™ Easy Match Universal Restorative.
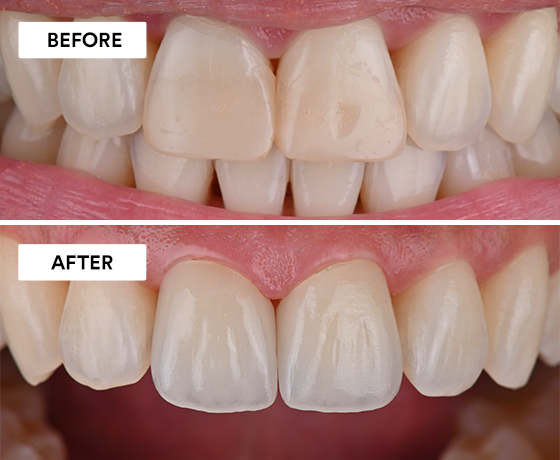
Using 3M™ Imprint™ 4 Light VPS Material, 3M™ Intra-oral Syringe, 3M™ Imprint™ 4 Heavy VPS Material, 3M™ Protemp™ 4 Temporization…
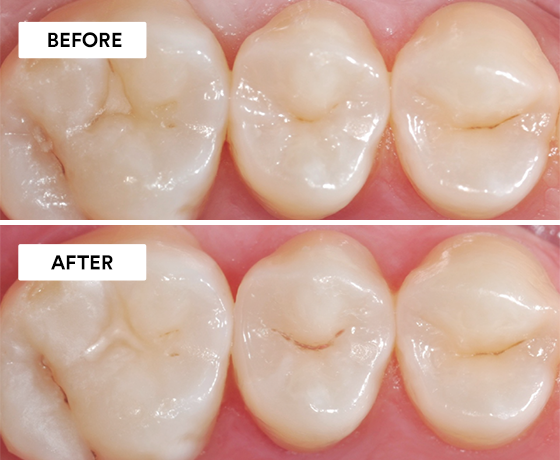
Using 3M™ Scotchbond™ Universal Etchant, 3M™ Scotchbond™ Universal Adhesive, 3M™ Elipar™ DeepCure-S LED Curing Light, 3M™ Filtek™ One Bulk Fill…